Osteoporosis and role rankl-rank-opg system and notch signaling pathway in bone development and remodeling
Summary. Physiological bone remodeling is a highly coordinated process responsible for bone resorption and formation and is necessary to repair damaged bone and maintain mineral homeostasis. In addition to the traditional bone cells, osteoblasts, osteoclasts and osteocytes, that are necessary for bone remodeling biological active factors have also been implicated in bone disorders. This review discussed in detail are the cellular and molecular mechanisms of bone remodeling, including events that orchestrate the role cytokine RANKL-RANK-OPG system and Notch signaling of bone remodeling and development of osteoporosis.
INTRODUCTION
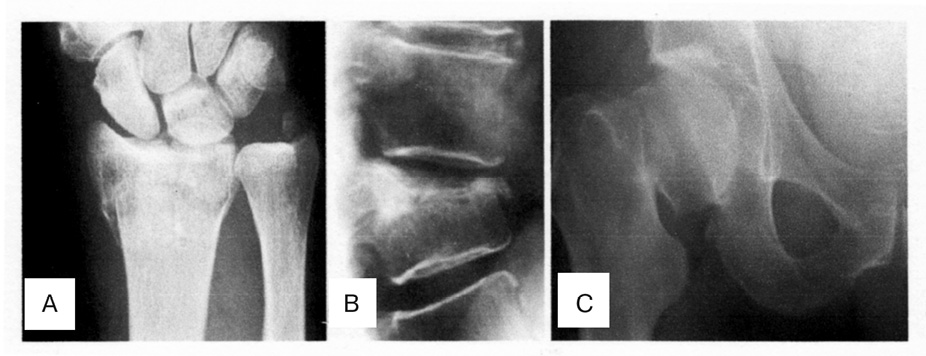
Osteoporosis (OP), a progressive systemic skeletal disorders characterized by low bone mineral density (BMD), deterioration of the microarchitecture of bone tissue, and increases risk for fracture. OP is a complex multifactoral disease, determined by genetic and environmental factors as well as their interactions. A lange number of molecular, genetic and environmental factors underlying OP have been indentified in past decades [62]. OP is defined by the World Health Organizations (WHO) as a BMD that is 2,5 standard deviation or more below the mean peak bone mass [64]. OP is becoming an escalating problem worldwide due to an increase in life expectancy and therefore in the ageing population. Currently it is estimated that over 200 million people worldwide suffer from this disease [14]. Approximately 30% of all postmenopausal women have OP in the United States and in Europe. Ageing of populations worldwide will be responsible for a major increase of the incidence of OP in postmenopausal women. The most significant outcome of low bone mass and deteriorated bone quality is fracture, which is most commonly at the hip, radius, and vertebra [16] (Figure 1). The estimated causes of OP in the United States alone are at 10 million, with another 34 million individuals at risk of fracture due to low bone mass. An estimated prevalence is that the number of persons older than age 50 with OP will increase to 12 million by the year 2010 and to nearly 14 million by the year 2020 [31]. The annual incidence of osteoporotic fractures exceeds 1.5 million in the U.S.A. Hip fractures are projected to increase to 6,3 million by the year 2050. The mortality rate in the patients with hip fracture is 1 in 5 persons during the first year after fracture [14]. Osteoporotic fractures have been estimated to cost the US health care system approximately 17 billion $ annually, with an annual cost projected to approach 50 billion $ by the year 2040; similarly, the total osteoporotic fractures — related direct costs in Europe are forecast to increase from 23,1 billion € in 2010 to 56 billion € by the year 2050 [26, 32]. Thus, the effective prevention and treatment of fragility fractures in the OP patients is very important in the current clinical practice worldwide. This article reviews the cellular participants and molecular mechanisms that coordinate bone remodeling and include an assessment of cytokine receptor activator of nuclear factor kappa B (NF-kB) ligand (RANKL) RANKL-RANK-OPG system and Notch signaling and their key role in regulating normal bone physiology and development of the OP.
CELLS INVOLVED IN BONE REMODELING: OSTEOBLASTS AND BONE FORMATION
Bone is a dynamic tissue that undergoes continual adaption during life to attain and preserve skeletal size, shape and structural integrity and regulate mineral homeostasis. Two processes, remodeling and modeling, underpin development and maintenance of the skeletal system. Bone modeling is responsible for growth and mechanically induced adaption of bone and requires that the process of bone formation and bone resorption, while globally coordinated, occur independently at distinct anatomical location. This tightly coordinated event requires the synchronized activities of multiple cellular participants to ensure bone resorption and formation occur sequentially at the same anatomical location to preserve bone mass. Bone remodeling is a physiological process that maintains the integrity of the skeleton by removing old bone and replacing it with a young matrix. Two principle cell types are found in bone, the osteoclast, and the osteoblast, which are the major effectors in the turnover of bone matrix [52, 56]. Osteoblasts and osteoclasts dictate skeletal mass, structure, and strength via their respective roles in resorbing and forming bone. Osteoblasts are specialized mesenchymal-derived cells whose function is the deposition and maintenance of skeletal tissue. Osteoblasts derive from pluripotent mesenchymal stem cells (MCS) that prior to osteoblast commitment can also differentiate into other mesenchymal cells lineages such as fibroblasts, chondrocytes, myoblasts and bone marrow stromal cells including adipocytes, depending on the activated signaling transcription pathways. Understanding the mechanisms that control the differentiation of osteoblastic cells from MCS is thus one of the fundamental areas of research of bone biology. Several specific transcription factors are responsible for the commitment of pluripotent MSC into the osteoblast cell lineage [2]. Lineage-specific gene expression is ultimately under the control of transcription factors that act to regulate specifi c gene expression. They act as the key switching mechanisms to induce gene transcription. Considerable progress has been made in identifying those transcription factors which act as «master switches» during commitment of multipotent cells to specific lineages. A major breakthrough in understanding genetic regulation of osteoblast differentiation was made with the identification of the role of the transcription factor core binding factor 1 (Cbfa-1/runt-related transcription factor-2 (RUNX-2)) [2, 34, 35]. Cbfa-1/RUNX-2 expression is an absolute requirement for osteoblast differentiation. In Cbfa-1 knockout mice there is a normal cartilaginous skeleton seen but a complete absence of bone formation [61, 63]. Cbfa-1/RUNX-2 known to interact directly with the osteocalcin promoter to induce its expression [12]. However an additional transcription factor, Osterix, which is a downstream target for Cbfa-1/RUNX-2, has also been shown to be an absolute requirement for normal osteoblast differentiation in knockout mice experiments [35]. More recent studies have shown the existence of distinct isoforms of Cbfa-1, which may have subtly different roles during normal tissue formation, including regulation of cartilage expression in addition to bone. Another runt-related gene that plays an important role in the commitment of multipotent MSC to the osteoblastic lineage and for osteoblast differentiation at an early stage is RUNX-2. Cbfa-1/RUNX-2 are involved in the production of bone matrix proteins [69], as it is able to up-regulate the expression of major bone matrix protein genes, such as type I collagen, osteopontin, bone sialoprotein and osteocalcin leading to an increase of immature osteoblasts from MCS; the immature osteoblasts from immature bone [35, 61]. Osteoblast commitment, differentiation and growth are controlled by several local and systemic factors that can also act in a paracrine and/or autocrine way and that can regulate the activity of specific transcription factor [12]. Huge advances have been made in the understanding of cellular and molecular control of bone formation in the past decade. The establishment of in vitro models of osteoblast differentiation and formation has been essential for determining the effects of specific growth factors and growth factor-induced transcription factors on osteogenesis. Osteoblasts play a crucial role in the process of bone formation, in the induction and regulation of extracellular matrix mineralization and in the control of bone remodeling [53]. During bone formation, mature osteoblasts synthesize and secrete type I collagen (which represents the greated part of the organic extracellular bone matrix) and various non-collagen proteins such as osteocalcin, osteopontin and bone sialoprotein (which exert various essential functions, including the regulation of bone turnover, the control of bone mineral deposition and regulation of bone cell activity). Osteocalcin (Gla) is a vitamin-K-dependent osteoblast-specific protein and whose synthesis is enhanced by 1.25 OH vitamin D3 and reflects metabolic cellular activity. Of the de novo synthesized osteocalcin, 60–90% is incorporated into the bone matrix where it binds to hydroxyapatite during matrix mineralization. Osteopontin (OPN) is a phosphorylated acidic glycoprotein that is present in large amounts in immature bone. OPN is synthesized by osteoblast but is expressed by other cellular types, such as chondrocytes; it is involved in various physiological and pathological events. Bone sialoproteins I glycosylated, phosphorylated and sulfated protein that promotes hydroxyapatite crystal nucleation and osteoblast differentiation [24]. This has been confirmed by the observation that bone-sialoprotein-knockout mice present hypo-mineralized bone, a reduction in the size of their long bones and aberrant levels of osteoblast markers [44]. Osteoblasts also synthesize cytokine interleukin (IL)-1 and IL-6, which control bone cells in an autocrine and/or paracrine manner. Various in vitro studies of human and murine osteoblastic cell lines suggest that IL-1 can affect proliferation, collagen and osteocalcin synthesis and alkaline phosphatase (Alp) production [41, 47]. Osteoblasts express receptors for various hormones including parathyroid hormone (PTH) [52], 1.25 (OH)2D3 [48], estrogenes [33], which are involved in the regulation of osteoblast differentiation and activity. Vitamin D3 is able to modulate the metabolic activity of osteoblasts through the activation of a series of Vitamin-D-responsive genes that reflect a more mature osteoblast phenotype.
CONTROL OF BONE REMODELING
BY OSTEOBLASTS:
THE ROLE RANKL-RANK-OPG SYSTEM OF THE OSTEOCLAST DEVELOPMENT
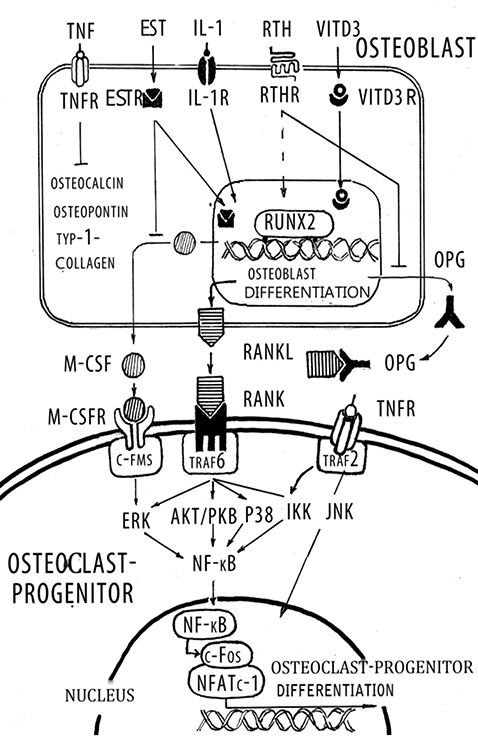
In recent years it has become evident that osteoblasts have a global role in orchestrating the bone remodeling process. Their function is not restricted solely to bone formation, but it is now firmly established that they are responsible for initiating bone resorption. In cellular terms, apart from forming the mineral and organic extracellular compartment of bone, the osteoblast provides the essential and sufficient stimuli that control the behavior of the osteoclast, an event that occurs via cell-cell interaction. The bone resorption cascade involves a series of steps directed towards the removal of both the mineral and organic constituents of bone matrix by osteoclasts, aided by osteoblasts. The role of the osteoclast as a major resorbing cell, and its structure and biochemical properties have been well characterized [4]. The first stage involves the recruitment and dissemination of osteoclast progenitors to bone. The progenitor cells are recruited from the haemopoietic tissue such as bone marrow and slenic tissue to bone via the circulating blood stream. They proliferate and differentiate into osteoclasts through a mechanism involving cell-to-cell interaction with osteoblast stromal cells. Osteoclast formation from osteoclast precursor is regulated predominantly by osteoblastic cells during normal bone remodeling. Osteoblastic cells in the bone marrow express two cytokines that are required for osteoclast-progenitor differentiation into osteoclasts: RANKL and osteoprotegerin (OPG) [55] (Figure 2). The discoveries of the RANKL and OPG have revolutionized our understanding of the process underlying osteoclast formation and activation [29, 60]. RANKL and OPG potently stimulate and inhibit, respectively, osteoclast differentiation. RANKL is a membrane bound factor that is produced by osteoblasts and stromal cells in response to a variety of signals such as PTH, tumor necrosis factor (TNF)-α and IL-1. RANKL bind to the cytoplasmic membrane receptor RANK (receptor activator of NF-kB), which is a member of the TNF receptor super family and subsequently induces both osteoclast differentiation and activation. OPG is a soluble decoy receptor for RANKL and can inhibit its effects, thereby preventing osteoclast development and subsequent bone resorption [6]. Over expression of OPG in transgenic mice results in osteopetrosis, and, conversely, OPG deficient mice exhibit severe OP. Many of the same agent that stimulate RANKL expression (including PTH, IL-1, prostaglandin E) also inhibit OPG expression [47], which enhances osteoclastogenesis even further. While fibroblast growth factor-2 induces RANKL expression by osteoblasts, it also inhibits osteoclast differentiation directly by interfering with the action of macrophage colony stimulating factor (M-CSF) [48]. In contrast, to the stimulatory effects of the agents described above, estrogen inhibits the production of RANKL by osteoblasts [33]. Transforming growth factor (TGF)-β also strongly suppresses RANKL expression by osteoblasts, whereas it stimulates OPG expression [8]. Administration of RANKL to mice causes OP, whereas disruption of the RANKL gene in mice leads to severe osteopetrosis, impaired tooth eruption, and the absence of osteoclasts [43]. Membrane bound M-CSF is also a critical early modulator in the differentiation of osteoclasts [40]. M-CSF binds to c- Fms on the surface of osteoclast precursors, and this event enhances their proliferation and survival. M-CSF enhances the survival of monocyte stem cells thereby permitting them to respond to direct inducers of differentiation such as RANKL. A combination of M-CSF and RANKL is sufficient for human, mouse, and rat multinucleated osteoclast formation in vitro [2]. Although RANKL is critical for osteoclast formation and activation, a series of complementary studies has revealed a number of additional gene products that are necessary for osteoclastogenesis and a variety of hormones and cytokines that modulate osteoclast formation [47, 30]. Deletion of the genes for M-CSF, c-fos, RANK and NF-kB results in absent osteoclast formation confirming their requirement for osteoclastogenesis. Osteoclasts are formed in mice whom the genes for TRAF6 and the c-fos have been deleted; however, these osteoclasts exhibit defects in bone resorption resulting in osteopetrosis [11]. Interestingly, another TRAF6 knockout mice exhibits defective osteoclastogenesis [25]. TRAF6 activates the MAP kinase cascade, and eventually activates JNK, JKK and N-kB have been directly implicated in the response to RANKL [11].Different domains of TRAF6 modulate both the initial differentiation and subsequent maturation of osteoclasts by activating various kinase cascades. RANKL also activates NF-kB in osteoclasts, in large part via TRAF stimulation of Ik kinase (IKK) to phosphorylate IkB, which then dissociates from NF-kB, and permits NF-kB translocation into the nucleus and subsequent binding to NF-kB responsive genes. TNF-α also acts to induce osteoclast formation and activation in concert with RANKL via the TNF receptor and TRAF2/6 and subsequently to activate NF-kB signaling [25].
OSTEOCLAST AND BONE RESORPTION
The development of an in vitro bone resorption model using isolated primary osteoclasts and mineralized bone matrix as a substrate almost twenty years ago provided an excellent system for detailed cell biological studies of bone resorption [58]. Although this model has several limitations in attempts to study the whole physiological cascade of bone resorption, it provides an excellent tool for detailed studies of the cellular mechanisms involved in the destruction of mineralized bone matrix. The sequence of cellular events needed for bone resorption is called the resorption cycle. Resorption requires cellular activates : migration of the osteoclast to the resorption site, its attachment to bone, polarization and formation of new membrane domains, dissolution of hydroxyapatite, degradation of organic matrix, removal of degradation products from the resorption lacuna, and finally either apoptosis of the osteoclasts or their return to the nonresorbing stage. The term resorption cycle covers neither the differentiation pathway nor the cellular activities needed for the fusion of mononuclear precursor to form the multinuclear mature osteoclast. It should not be mistaken for the more widely used term remodeling cycle, which is used to describe the bone remodeling at the tissue level that involves the activities of several different cell types. After migration of the osteoclast to a resorption site, a specific membrane domain, the sealing zone, forms under the osteoclast. The plasma membrane attached tightly to the bone matrix and seals the resorption site form its surroundings.The molecular interactions between the plasma membrane and the bone matrix at the sealing zone is still unknown. Several lines of evidence have shown, however, that integrins play an important role in early phases of the resorption cycle [70]. At last four different integrins are expressed in osteoclasts: αvβ3, αvβ5, α2β1 and αvβ1 [38]. The role of αvβ3 has received much attention, because antibodies against αvß3, as well as argynine-glycine-aspartic acid (RGD)-containing peptides such as echistation and kistrin, are defective inhibitors of bone resorption both in vitro and in vivo [17]. αvβ3 is highly expressed in osteoclasts and is found but what the plasma membrane and in various intracellular vacuoles. However, the precise function of αvβ3 in resorbing osteoclasts remains unknown; the integrin could play a role both in adhesion and migration of osteoclasts and in endocytosis of resorption products. The latter possibility is supported by the observation that high amount of αvβ3 are present at the ruffled border and by recent data from receptor-binding assays showing that denatured type I collagen has a high affinity for αvβ3 [70]. Some authors have suggested that αvβ3 integrin also mediates the attachment of the sealing zone to the bone matrix [70]. Previous ultrastructural studies indicated that resorbing osteoclasts are highly polarized cells [5]. Current data suggest that resorbing osteoclasts contain not only the sealing zone but also at least three other specialized membrane domains: a ruffled border, a functional secretary domain and a basolateral membrane [52, 58]. As the osteoclast prepares to resorb bone, it attaches to the bone matrix through the sealing zone and forms another specific membrane domain, the ruffled border. The ruffled border is a resorbing organelle, and it is formed by fusion of intracellular acidic vesicles with the region of plasma membrane facing the bone [58]. During this fusion process much internal membrane is transferred, and forms long, finger-like projections that penetrate the bone matrix. The characteristics of the ruffled border to not match those of any other plasma membrane domain described. Although facing the extracellular matrix, it has several features that are typical of late endosomal membranes. Several late endosomal markers, such as CIC-7, V-type H+-ATPase, are densely concentrated at the ruffled border [51]. The main physiological function of osteoclast is degrading mineralized bone matrix. This involves dissolution of crystalline hydroxyapatite and proteolytic cleavage of the organic matrix, which is rich in collagen. Before proteolytic enzymes can reach and degrade collagenous bone matrix, tightly packed hydroxyapatite crystals must be dissolved. It is now generally accepted that the dissolution of mineral occurs by targeted secretion of HCl through the ruffled border into the resorption lacuna. This is an extracellular space between the ruffled border membrane and the bone matrix, and is sealed from the extracellular fluid by the sealing zone. The low pH in the resorption lacuna is achieved by the action of ATP-consuming vacuolar proton pumps both at the ruffled border membrane and in intracellular vacuoles. Osteoclasts attach to bone and form a circumferential sealing zone that isolates the bone resorption compartment from the extracellular space. Osteoclast plasma membrane within the sealing zone develops into the ruffled border. The observation that NH4Cl reversibly inhibits bone resorption by osteoclasts indicates that the resorption compartment is acidic and that the sealing zone is impairment to H+ and NH+4. The osteoclast cytoplasm is rich in carbonic anhydrase II (CA II) [20], proving a continuous supply of protons and bicarbonate. Protons are transported across this membrane into the bone resorption compartment by vacuolar-type H+-ATPase (V-type ATPase) [51]. Chloride ions passively follow the protons through conductive anion channels [50]. The combined activities of the proton pump and chloride channel acidify the resorption compartment and alkalinize the cytoplasm. Bicarbonate exits the cell into the extracellular space in exchange for chloride via a basolateral electroneutral anion exchanger, correcting the cytoplasmic alkalinization and compensating for cytoplasmic chloride loss. The net result of these coordinated transport activities is the transcellular movement of HCl into the bone resorption compartment. This model predicts that both the ruffled border proton pump and chloride channel play key roles in bone resorption. The proton pump provides the proton-motive force necessary to generate a pH gradient. However, the pump is electrogenic. The chloride channel shot-circuits the electrogenic pump and allows maximal proton transport. It follows that limitation of the chloride conductance could inhibit acid transport independently of the intrinsic activity of the proton pump. Analogous to a current model for regulation of the pH of some intracellular organelles, regulation of the anion conductance rather than proton pump activity could be the key point at which the rate of osteoclast acid transport, and hence bone resorption, is governed. Thus, molecular characterization of the ruffled border chloride channel may provide insight into regulation of osteoclast bone resorption and could define a pharmacological target for the treatment of metabolic bone disease [4]. The osteoclast proton pump is sensitive to bafilomycin A1, which also effectively inhibits bone resorption both in vitro and in vivo. The recent finding that vacuolar ATPase at the ruffled border contains cells specific subunits has further encouraged development of resorption inhibitors that inhibit the osteoclast proton pump. Protons for the proton pump are produced by cytoplasmic carbonic anhydrase II, high levels of which are synthesized in osteoclasts. In order to generate protons, the presence of CA II is essential. It catalyzes the conversion of H2O and CO2 into H2CO3, which then is ionized into H+ and HCO–3 [49]. Mutation in CA II can cause osteopetrosis due to non-functional osteoclasts [45]. The HCO–3 ions are exchanged for Cl– through an anion exchanger, membrane transport protein AE2, located in the basolateral membrane, leading to continued of Cl– for acidification of the resorption lacuna [46]. After solubilization of the mineral phase, several proteolytic enzymes degrade the organic bone matrix, although the detailed sequence of events at the resorption lacuna is still obscure. Two major classes of proteolytic enzymes, lysosomal cysteine proteinases and matrix metalloproteinases (MMPs) have been studied most extensively. Osteoclasts produce proteases, of which cysteine proteinase cathepsin K has prevent to be the most important [23], aiding the degradation of the organic bone matrix. Eleven different types have been described (B, C, F, H, K, L and other) with cathepsin K being the most important with respect to bone remodeling, since it is a protease with intense collagenase activity, especially with respect to acid pH, which is essential to dissolve calcic hydroxyapatite, the main mineral component of bone. It degrades the two types of collagen, I and II and is predominantly expressed in osteoclasts [39]. Cathepsin K gives rise to specific degradation products-like C-terminal cross-linking telopeptide of type I collagen (CTX-I), which can be used for measurements of bone resorption [10]. The role of cathepsin K in bone resorption was determined using evidence from an autosomal recessive osteochondrodysplasia named pycnodysostosis, a very rare disease characterized by high BMD, acroosteolysis of the distal phalanxes, shot stature, and cranial deformaties with late closing of the fontanelles [66]. Studies in mice submitted to nonfunctional mutations of cathepsin have given rise to different models of osteopetrosis [23]. Matrix in bone resorption [20], during which, MMP activity is known to give rise to a specific degradation fragment, C-terminal telopeptide of type I collagen (ICTP) [7]. After matrix degradation, the degradation products are removed from the resorption lacuna through a transcytotic vascular pathway from the ruffled border to the functional secretory domain, where they are liberated into the extracellular space. Quantitative data are still missing, but clear large amounts of degraded extracellular material must be transported through the resorbing cell, given that the volume of the resorption pit can easily exceed the volume of the entre cell. The extent to which the degradation of collagen and other matrix components is extracellular and the extent to which this takes place in intracellular transcytotic compartments are not known. Recent results have suggested that tartrate-resistant acid phosphatase (TRAP), a widely used osteoclast marker, is licalized in the transcytotic vesicles of resorbing osteoclasts, and that it can generate highly destructive reactive oxygen species able to destroy collagen. This activity, together with the co-localization of TRAP and collagen fragments in transcytotic vesicles, suggests that TRAP functions in further destruction of matrix-degradation products in the transcytotic vesicles. The observed mild osteopetrosis in TRAP-knockout mice support this hypothesis [7].
NOTCH ROLE IN BONE DEVELOPMENT AND REMODELING
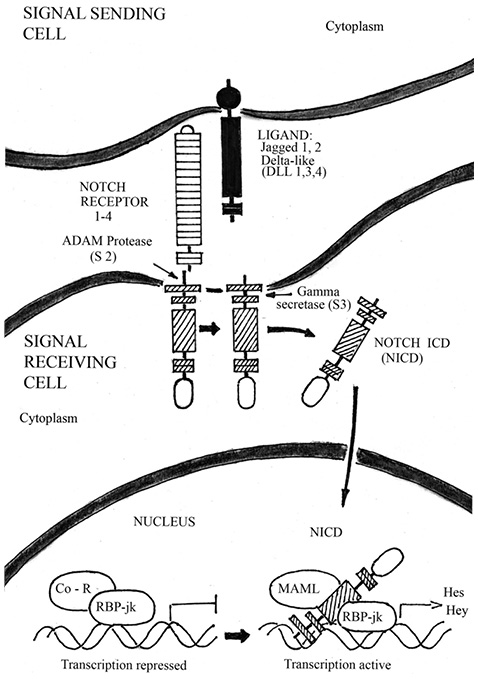
Bone is a dynamic tissue, undergoing a continual remodeling process involving a cycle of formation of new bone tissue and breakdown (resorption) of older bone tissue. In OP, the balance of these processes is tipped toward resorption, leading to weakening of bone tissue and increased risk of fracture [54]. Recent progress in OP research has suggested that decreased BMD is the result of an active process of osteogenic differentiation, induced by a resorption response. At molecular level, the bone development and remodeling process is regulated by a network of signaling pathways, including Wnt/β-catenin — BMPs pathways, and Notch signaling, which control the master regulator of osteogenesis Cbfa 1/ RUNX2. The canonical Notch signaling pathway, implicated in skeletal development and disease, consist of five ligands (Delta-like ligand 1, 3, 4 and Jagged 1, 2) that interact with four Notch receptors (Notch 1–4) (Figure 3). Since Notch receptors and their ligands are transmembrane proteins, cell-cell interaction is required for activating Notch signaling cascades [19]. Notch proteins can be divided into three parts: an extracellular domain, a transmembrane segment, and Notch intracellular domain (NICD) [36]. Upon ligand binding, the Notch receptor is cleaved, first by TNF-α conversion enzyme (ADAM) and then subsequently by the gamma secretase complex that consist of Presenilin 1 and 2 in mammals. Upon receptor cleavage, the NICD translocates to the nucleus where it binds to the transcription factor recombination signal binding protein for immunoglobulin kappa j (RBP-jk) to regulate the expression of the target genes. This binding, along with the co-activator Mastermind-like (MAML), result in a switch from a transcriptional repressor complex to an activation complex. Together, this binding complex ultimately activates expression of downstream target genes such as basic helix-loop-helix transcriptional repressors related to Hairy enhancer of split (Hes 1, 5, 7), or to Hes-related with YRPW motif (Hey 1, 2 and L), to affect many cellular processes including cell proliferation and differentiation [37]. While the NICD protein has the ability to function in an RBP-jk-independent manner, non-canonical Notch signaling has yet to be implicated in skeletal development. Notch signaling has emerged as an important regulator of skeletogenesis with multiple roles in chondrogenesis, osteoblastogenesis and osteoclastogenesis. In addition, Notch pathway manipulation in the osteogenic lineages in vivo demonstrates that Notch inhibits osteogenic differentiation and growth in skeleton. In this chapter, we will discuss Notch pathway regulation of normal skeletal development and its role in bone diseases such as OP and osteopetrosis.
NOTCH INHIBITION OF OSTEOBLAST DIFFERENTIATION
Bone is mineralized connective tissue that is the main component of the skeletal system and provides strength and support for the body. There are two types of bone formation, endochondral ossification (described above) and intramembranous ossification, which forms the flat bones of the body and involves the differentiation of cells within the mesenchymal condensations directly into bone [13]. Osteoblasts, or bone forming cells, share a common progenitor with chondrocytes and are also derived from multipotent mesenchymal cells [9]. After osteoblast lineage induction, the precursor population proliferates to expand and subsequently undergoes maturation and finally mineralization. Residing near the bone surface, osteoblast precursor cells proliferate and commit to the osteoblast lineage. These pre-osteoblasts undergo matrix maturation through the expression of early markers of osteogenic differentiation including Col1a1, Alp and Runx2 and subsequently mineralize and express late makers of osteoblast differentiation including osteocalcin (Ocn) [13]. Mature and functional osteoblasts, or osteocytes, provide mechanical support and regulate mineral deposition. Notch regulation of osteoblast commitment and differentiation has been well documented. In vitro studies show that Notch signaling suppresses osteoblastic differentiation through the inhibition of osteogenic markers Alp, Ocn, Col1a1 and Runx2, resulting in suppression of calcification [1, 67]. In stromal cells, NICD signaling inhibits osteoblastogenesis by Hes1-mediated suppression of Wnt/β-catenin signaling, indicating an antagonistic relationship between the pathways in osteogenesis [15, 27, 42]. Furthermore, repression of osteoblast differentiation is also mediated through Notch1 itself or by Notch downstream effectors Hes1 and Hey1 binding directly to and inhibiting Runx2 transcriptional activity [18, 27, 28]. Runx2 is considered the master regulator of osteoblast differentiation. Forced expression of Runx2 in non-osteoblast cells is sufficient to induce the expression of many osteoblast genes while Runx2 null mice lack osteoblasts, resulting in defective osteoblast differentiation and no endochondral or intramembranous bone formation. Thus, Notch-mediated repression of Runx2 transcriptional activity provides evidence for a direct mechanisms osteoblastogenesis inhibition [1]. In disagreement, two studies show that increased Notch signaling in MC3T3 cells stimulates osteoblast differentiation through the induction of calcific nodules. It is likely that the differing results could be due to cell culture conditions or the cell lines utilized and that the timing and levels of Notch signaling determine its effects on osteogenic gene induction. While there have been controversial in vitro results, recent in vivo data have helped clarify the role of Notch signaling in osteoblastogenesis [18] Loss of Presenilin1 and Presenilin2 in the osteoblast lineage results in overall loss of bone mass and age-related OP. Over-expression of NICD under the control of the 3.6kb Col1a1 promoter, expressed in early osteoblast precursors, results in runting from an overall decrease of bone volume leading to osteopenia. NICD over-expression with the 2.3kb Col1a1 promoter, expressed in mature osteoblasts, also results in skeletal dysfunction with progressive growth retardation, but due to increased proliferation of immature osteoblasts leading to increased bone mass and osteosclerosis. In this setting, Notch inhibits terminal osteoblast differentiation of committed progenitors, which allows for proliferation of immature osteoblasts. Together, activation of NICD inhibits osteoblast differentiation in both cases, but the resulting phenotype is dependent on the time of inhibition. Loss of Notch1 alone or of Notch1 and Notch2 together in differentiated osteoblasts did not result in skeletal abnormalities, indicating that Notch does not regulate mature osteoblast homeostasis, but inhibits osteoblast precursor differentiation during development [18]. Collectively, these in vitro and in vivo studies illustrate Notch inhibition of osteoblast differentiation at multiple stages of osteoblastogenesis.
NOTCH PATHWAY SUPRESSION OF OSTEOCLASTOGENESIS
Osteoclasts are derived from hematopoietic cells of the monocyte/macrophage lineage and provide the unique function of bone resorption. In conjunction with bone producing osteoblasts, osteoclasts maintain the skeletal system in homeostasis. Osteoclastogenesis commences when a subset of macrophages commit to the osteoclast lineage, proliferate, differentiate and reabsorb bone. After osteoclast lineage determination, M-CSF is required for pre-osteoclast proliferation and survival. However, the differentiation of osteoclasts is essentially regulated by signaling interaction with osteoblasts. Osteoblasts express M-CSF and RANKL to promote macrophage commitment to the osteoclast lineage. RANKL promotes osteoclastogenesis through the stimulation of a transcription factor complex including NFATc1, while also later promoting bone resorption through the induction of a bone reabsorbing complex including the RANK receptor. Similar to RANKL, OPG is produced by osteoblasts and competes with the RANK receptor for RANKL, acting as a decoy receptor effectively modulating osteoclast production. Therefore, the balance between the osteoclast stimulator, RANKL and the osteoclast inhibitor, OPG, determines the amount and rate of osteoclast production. Notch signaling negatively regulates osteoclastogenesis as demonstrated by in vitro and in vivo manipulation of Notch signaling directly in osteoclasts and indirectly in osteoblasts [3]. Activated Dll1 inhibits osteoclast formation in hematopoietic cells, while constitutively active Notch1 reduces M‑CSF and enhances RANKL and OPG gene expression, resulting in an overall reduction of osteoclast formation in stromal cells [21]. In a further study constitutively active NICD expression in mesenchymal cell lines inhibits osteoclastogenesis via inhibition of RANKL expression. In agreement, Jagged1 inhibits osteoclastogenesis in bone marrow macrophages while Notch1 and Notch3 loss of function in vivo in the osteoclast lineage directly promotes osteoclast formation with increased cell proliferation [3]. Furthermore, loss of Notch1 in the osteoblast lineage increases RANKL expression and decreases expression of OPG, therefore, indirectly promoting osteoclast formation [3]. In addition, conditional loss of Presenilin1 and Presenilin2 in the osteoblast lineage results in increased osteoclasts due to a reduction of OPG expression, leading to OP [18, 68]. Overall, these studies show that Notch signaling negatively regulates osteoclast formation and proliferation. Recently, some controversy on the role of Notch signaling in osteoclastogenesis has arisen with a study showing that RANKL has the potential to induce Jagged1 and Notch2 in bone marrow macrophages, while loss of Notch signaling or introduction of Notch2 shRNA suppresses RANKL-induced osteoclastogenesis. Likewise, Notch2 was shown to bind to the NFATc1 promoter and drive its expression, also resulting in increased osteoclast formation [22]. It is plausible that Notch signaling regulates multiple stages of osteoclastogenesis to either activate or repress osteoclast formation and activity. However, additional studies a required to reconcile these differing roles of Notch signaling in osteoclastogenesis.
CONCLUSION
In vitro and in vivo studies support a role for the Notch pathway in osteoblastogenesis, osteoclastogenesis and bone formation. However, the direction (inhibitory or stimulatory) of the effect of Notch signaling on osteoblastogenesis is controversial. Loss of Notch signaling in osteoblasts may lead to OP through activation of osteoclastogenesis [18, 68]. In contrast, suppression of Notch signaling by a selective γ-secretase inhibitor or Notch short hairpin RNA suppresses RANKL-induced osteoclastogenesis, whereas ectopic expression of intracellular Notch promotes osteoclastogenesis [66]. Interestingly, a genome-wide association study identified JAG1 as a candidate gene for BMD regulation and a potential risk factor for fracture [38]. These findings are concordant with the increased risk for pathologic fractures observed in individuals with OP. Further investigations are needed to understand the mechanisms by which OP may be caused by Notch dysfunction. Finally, new findings establish an important role for Notch signaling in bone homeostasis that may lead to better understanding of the mechanisms contributing to OP. Advances understanding the role of Notch in various cells of the skeleton have provided important insights into the etiology of skeletal conditions like osteosclerosis and OP. Attempts to modulate Notch signaling pathways have been made to treat disease such as OP in various mouse models. To translate these results into therapeutic reality, toxicity needs to be considered in terms of usage of gamma-secretase inhibitors or pan-Notch receptor antibodies, which have demonstrated high intestinal toxicity in rodents [65]. Antibodies that recognize the Notch negative regulatory region and antagonize individual Notch receptors may reduce or limit this adverse effect [59, 65]. Ligand-specific antibodies against Dll1 and Dll4 further reduce the toxicity in treating a mouse model of graft-versus-host disease [59], suggesting a rational design of ligand neutralizing antibodies specific for targeted tissues, which may shed promising light on its application. The skeletal defects exhibited by Dll3 and Jag2 null mice and human skeletal diseases caused by mutations in DLL3 and JAG1 underscore the specific utilization of the Notch ligands in the context of skeletogenesis. Hence, defining functions and the molecular mechanisms of Notch ligands will allow us to delineate the signal-sending partners of the Notch pathway and to develop ligand-targeted therapy for skeletal disorders. Distinguishing canonical vs. non-canonical Notch signaling is the second concern. Increasing evidence suggests that RBP-jk-independent Notch signaling governs differential responses to Notch stimulation (at least under pathological conditions). In conclusion, Notch signaling regulates multiple steps of osteoblast development including osteoblast commitment, proliferation and maturation as well as functional interaction with osteoclasts and HSCs in the bone microenvironment.
REFERENCES
1. Ann E.-J., Kim H.-J., Choi Y.-H. et al. (2011) Inhibition of Notch 1 signaling by RUNX2 during osteoblast differentiation. JBMP, 26(2): 317–330.
2. Augello A., De Bari C. (2010) The regulation of differentiation in mesenchymal stem cells. Human Gene Ther., 21(10): 1226–1238.
3. Bai S., Kopan R., Zou W. et al. (2008) Notch 1 regulates osteoclastogenesis directly in osteoclast precursor and indirectly via osteoblast lineage cells. J. Biol. Chem., 283(10): 6509–6518.
4. Boyce B.F., Rosenberg E., De Papp A.E. et al. (2012) The osteoblast, bone remodeling, and treatment of metabolic bone disease. Eur. J. Clin. Invest., 42(12): 1332–1341.
5. Boyce B.F., Yao Z., Xing L. (2009) Osteoclasts have multiple roles in bone in addition bone resorption. Crit. Rev. Eukaryot Gene Expr., 19(3): 171–180.
6. Boyce B.F., Xing L. (2007) Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther., 9(1): S.1.
7. Brömme D., Wilson S. (2011) Role cysteine cathepsin K in extracellular proteolysis. In: Extracellular matrix degradation, biology of extracellular matrix, 2th edn., Parks W.C., Mecham R.P. (Еds). Springer-Verlag, Heidelberg: 23–51.
8. Chen G., Deng C., Li Y.-P. (2012) TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci., 8(2): 272–288.
9. Chen S., Lee B.H., Bae Y. (2014) Notch signaling in skeletal stem cells. Calcif. Tissue Int., 94(1): 68–77.
10. Civitelli R., Armamento-Villareal R., Napoli N. (2009) Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporosis Int., 20(6): 843–851.
11. Darnay B.G., Besse A., Poblenz A. et al. (2007) TRAFs in RANKL signaling.
12. Datta H.K., Ng W.F., Walker J.A. et al. (2008) The biology of bone metabolism. J. Clin. Pathol., 61(5): 577–587.
13. 13.Deng Z.L., Sharff K.A., Tang N. et al. (2008) Regulation of osteogenic differentiation during skeletal development. Front Biosci., 13: 2001–2021.
14. Dennison E.M. (2011) Osteoporosis in 2010: building bones and (safely) preventing breaks. Nat. Rev. Rheumatol., 7(1): 80–82.
15. Deregowski V., Gazzerro E., Priest L. et al. (2006) Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/beta-catenin but not bone morphogenetic protein signaling. J. Biol. Chem., 281(10): 6203–6210.
16. Dhanwal D.K., Dennison E.M., Harvey N.C. et al. (2011) Epidemiology of hip fracture: worldwide geographic variation. Indian J. Orthop., 45(1): 15–22.
17. Dossa T., Arabian A., Windle J.J. et al.(2010) Osteoclast-specific inactivation of the integrin-linked kinase (IKL) inhibits bone resorption. J. Cell Biochem., 110(4): 960–967.
18. Engin F., Yao Z., Yang T. et al. (2008) Dimorphic effects of Notch signaling in bone homeostasis. Nat. Med., 14(3): 299–305.
19. Fiuza U-M., Arias A.M. (2007) Cell and molecular biology of Notch. J. Endocrinol., 194(3): 459–474.
20. Fujisaki K., Tanabe N., Suzuki N. et al. (2007) Receptor activator of NF-kappa B ligand induced expression of carbonicanhydrase II, cathepsin K, and matrix metalloproteinase-9 in osteoclast precursor RAW 264-7 cells. Life Sci., 30(4): 1311–1318.
21. Fukushima H. (2010) Regulatory mechanisms of Notch signaling in osteoclast differentiation. J. Oral Biosciences, 52(3): 205–214.
22. Fukushima H., Nakao A., Okamoto F. et al. (2008) The association of Notch2 and NF-kappa B accelerates RANKL-induced osteoclastogenesis. Mol. Cell Biol., 28(20): 6402–6412.
23. Garcia R.R., Munoz-Torres M. (2008) Cathepsin K: biological aspects and therapeutic possibilities. Med. Clinica, 131(6): 218–220.
24. Gordon J.A., Tye C.E., Sampaio A.V. et al. (2007) Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone, 41(3): 462–473.
25. Ha H., Han D., Choi J. (2009) TRAF- mediated TNFR-family signaling. Curr. Protoc. Immunol., 87(11): 1–11.
26. Hadji P., Klein S., Gothe H. et al. (2013) The epidemiology of osteoporosis — Bone Evaluation Study (BEST): an analysis of routine health insurance data. Dtsch. Arztebl. Int., 110(4): 52–57.
27. Hilton M.J., Tu X., Wu X. et al. (2008) Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat. Med., 14(3): 306–314.
28. Hodge J.-M., Collier F.M., Pavlos N.J. et al. (2011) M-CSF potently augments RANKL-induced resorption activation in mature human osteoclasts. PLoS ONE., 6(6): e 21462.
29. Hofbauer L., Rachner T. (2010) Die Rolle des RANK-RANKL-OPG-Signalwegs in Knochenstoffwechsel. Vorbildung Osteologie, 3(5): 118–121.
30. Imai Y., Kondoh S., Kouzmenko A. et al. (2010) Minireview: osteoprotective action of estrogens is mediated by osteoclastic estrogen receptor-alpha. Mol. Endocrinol., 24 (5): 877–885.
31. IOF World Congress on Osteoporosis and 10th European Congress of Clinical and Economic aspects of Osteoporosis and Osteoarthritis (2010) Osteoporosis Int., 21 (5): S1–S6.
32. Kanis J.A., McCloskey E.V., Johansson H. et al. (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis Int., 24(1): 23–57.
33. Komm B., Cheskis B., Bodine P.V.N. (2008) Regulation of bone cell function by estrogens. In: Fundamentals of osteoporosis, 3rd edn. Marcus R., Feldman D., Nelson D.A., Rosen C.J. (Еds). Academic Press, San Diego, р. 345–385.
34. Komori T. (2010) Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res., 339 (1): 189–195.
35. Komori T. (2011) Signaling networks in RUNX2-dependet bone development. J. Cell Biochem., 112(3): 750–755.
36. Kopan R., Ilagan M.X. (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell, 137(2): 216–233.
37. Kovall R.A. (2008) More complicated that it looks: assembly of Notch pathway transcription complexes. Oncogene, 27(28): 5099–5109.
38. Kung A.W.C., Xiao S-M., Cherny S. et al. (2010) Association of Jag1 with bone mineral density and osteoporotic fractures: a genome-wide association study and follow-up replication studies. Am. J. Hum. Genet., 86(2): 229–239.
39. Lecaille F., Brömme D., Lalmanach G. (2008) Biochemical properties and regulation of cathepsin K activity. Biochimie, 90(2): 208–226.
40. Lee M.S., Kim H.S., Yeon J.-T. et al. (2009) GM-CSF regulates fusion of mononuclear osteoclasts into bone-resorbing osteoclasts by activating the Ras/ERK pathway. J. Immunol., 183 (4): 3390–3399.
41. Lee Y.-M., Fujukado N., Manaka H. et al. (2010) IL-1 plays an important role in the bone metabolism under physiological conditions. Int. Immunol., 22(10): 805–816.
42. Lin G.L., Hankenson K.D. (2011) Integration of BMP, Wnt and Notch signaling pathways in osteoblast differentiation. J. Cell Biochem., 112(12): 3491–3501.
43. Lo I.N., Blair H.C., Poliani P.L. et al. (2012) Osteopetrosis rescue upon RANKL administration to RANKL (-/-) mice: a new therapy for human RANKL- dependent ARO. J. Bone Miner. Res., 27(12): 2501–2510.
44. Malaval L., Wade-Gueye N.M., Boudiffa M. (2008) Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J. Exp. Med., 205(5): 1145–1153.
45. 45.Margolis D.S., Szivek J.A., Lai L.-W. et al. (2008) Phenotypic characteristics of bone carbonic anhydrase II-deficient mice. Calcif. Tissue Int., 82(1): 66–76.
46. Morgan P.E., Pastorehava S., Staut-Tilley A.K. et al. (2007) Interaction of transmembrane carbonic anhydrase, CAIX, with bicarbonate transporter. Am. J. Physiol. Cell Physiol., 293 (2): 738–748.
47. Mundy G.R., Oyajobi B., Gutierrez G. et al. (2008) Cytokines in bone remodeling In: Fundamentals of osteoporosis, 3rd end. Marcus R., Feldman D., Nelson D.A., Rosen C.J. (Еds). Academic Press, San Diego, р. 453–490.
48. Neve A., Corrado A., Contatore F.P. (2011) Osteoblast physiology in normal and pathological conditions. Cell Tissue Res., 343(2): 289–302.
49. Nishita T., Tomita Y., Imanari T. et al. (2011) Biochemical and developmental characterization of carbonic anhydrase II in form chicken erythrocytes. Acta Vet. Scand., 53(1): 16–25.
50. Okamoto F., Kajiya H., Toh K. et al. (2008) Intracellular CIC-3 chloride channels promote bone resorption in vitro through organelle acidification in mouse osteoclasts. Am. J. Physiol. Cell Physiol., 294(3): 693–701.
51. Qin A., Cheng T.S., Pavlos N.J. et al. (2012) V-ATPase in osteoclasts: structure, function and potential inhibitors bone resorbtion. Int. J. Biochem. Cell Biol., 44(9): 1422–1425.
52. Raggatt L., Partridge N.C. (2010) Cellular and molecular mechanisms of bone remodeling J. Biol. Chem., 285(33): 25103–25108.
53. Rosenberg N., Rosenberg O., Soudry M. (2012) Osteoblasts in bone physiology: mini review. RMMJ., 3(2): e0013.
54. Sagalovsky S. (2012) Osteoporosis: cellular-molecular mechanisms of development and the target molecule to search for new treatments for diseases. Osteoporosis Osteopathy, 1: 15–22.
55. Sagalovsky S. (2013) Bone remodeling: cellular-molecular biology and cytokine RANK-RANKL-Osteoprotegerin (OPG) system and growth factors. Crim. J. Exptl. Clin. Med., 3(1–2): 36–44.
56. Sims N.A., Gooi J.H. (2008) Bone remodeling: multiple cellular interaction required for coupling of bone formation and resorption. Semin. Cell. Dev. Biol., 19(5): 444–451.
57. Ström O., Borgström F., Kanis J.A. et al. (2011) Osteoporosis: burden, health care provision and opportunities in the EU. Arch. Osteopor., 6(1–2): 59–155.
58. Tanaka S. (2008) Osteoclasts. IBMS Bone Key, 5(11): 454–457.
59. Tran I.T., Sandy A.R., Carulli A.J. et al. (2013) Blockade of individual Notch ligands and receptor controls graft-versus-host disease. J. Clin. Invest., 123(4): 1590–1604.
60. Trouvin R.-P., Goeb V. (2010) Receptor activator of nuclear factor-kB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin. Intervent Aging, 5(6): 345–354.
61. Tu Q., Zhang J., James L. et al. (2007) Cbfa 1/ RUNX2- deficiency delays bone wound healing and locally delivered Cbfa1. Wound Repair Regen., 15 (3): 404–412.
62. Umland E.M. (2008) An update on osteoporosis epidemiology and bone physiology. Univer. Tennessee Adv. Stud. Pharmacy, 5(7): 210–214.
63. Wojtowicz A.M., Templeman K.L., Hutmacher D.W. et al. (2010) RUNX2 over expression in bone marrow stromal cells accelerates bone formation in criticalsized femoral defects. Tissue Engineering Part A, 16(9): 2795–2808.
64. World Health Organization (2007) Assessment of fracture risk and its application to screening for postmenopausal women. WHO Technical Report. WHO, Genev.
65. Wu Y., Cain-Hom C., Choy L. et al. (2010) Therapeutic antibody targeting of individual Notch receptors. Nature, 464(7291): 1052–1057.
66. Xue Y., Cai T., Shi S. et al. (2011) Clinical and animal research findings in pycnodysostosis and gene mutation of cathepsin K from 1996 to 2011. Orhanet J. Rare Dis., 6(1): 20–30.
67. Zanotti S., Canalis E. (2010) Notch and the skeleton. Mol. Cell Biol., 30(4): 886–896.
68. Zanotti S., Smerdel-Ramoya A., Stadmeyer L. et al. (2008) Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology, 149(8): 3890–3899.
69. Ziros R.G., Basdra E.K., Papavassilion A.G. (2008) RUNX2: of bone and stretch. Int. J. Biochem. Cell Biol., 40(9): 1659–1663.
70. Zou W., Teitelbaum S.L. (2010) Integrins, growth factors, and the osteoclast cytoskeleton. Ann. N. Y. Acad: Sci., 1192: 27–31.
ОСТЕОПОРОЗ І РОЛЬ
RANKL-RANK-OPG-СИСТЕМИ
І NOTCH-СИГНАЛЬНОГО ШЛЯХУ У РОЗВИТКУ І РЕМОДЕЛЮВАННІ КІСТКИ
Резюме. Фізіологічно ремоделювання кістки становить високоскоординований процес резорбції та утворення нової кісткової тканини, процес, необхідний для відновлення пошкодження і підтримання мінерального гомеостазису. Цей процес здійснюється за участю традиційних кісткових клітин — остеобластів, остеокластів і остеоцитів, а також біологічних активних факторів, порушення співвідношення яких спричиняє розвиток кісткової патології. У наведеному огляді обговорюються клітинні й молекулярні механізми ремоделювання кістки, включаючи з’ясування ролі RANKL-RANK-OPG-цитокінової системи і Notch-сигнального шляху у регулюванні зазначеного процесу і розвитку остеопорозу.
Ключові слова: остеопороз, ремоделювання кістки, RANKL-RANK-OPG цитокінова система, Notch-сигнальний шлях.
ОСТЕОПОРОЗ И РОЛЬ
RANKL-RANK-OPG-СИСТЕМЫ
И NOTCH-СИГНАЛЬНОГО ПУТИ В РАЗВИТИИ И РЕМОДЕЛИРОВАНИИ КОСТИ
Резюме. Физиологически ремоделирование кости представляет собой высокоскоординированный процесс резорбции и образования новой костной ткани, процесс, необходимый для восстановления повреждения и поддержания минерального гомеостазиса. Этот процесс осуществляется с учетом традиционных костных клеток — остеобластов, остеокластов и остеоцитов, биологически активных факторов, нарушение соотношения которых способствует развитию костной патологии. В настоящем обзоре обсуждаются клеточные и молекулярные механизмы ремоделирования кости, включая выяснение роли RANKL-RANK-OPG-цитокиновой системы и Notch-сигнального пути в регулировании данного процесса и развития остеопороза.
Ключевые слова: остеопороз, ремоделирование кости, RANKL-RANK-OPG-цитокиновая система, Notch-сигнальный путь.
Correspondence:
Stanislav Sagalovsky, Dr. med.
E-mail: s.sagalovsky@gmail.com

Leave a comment